|
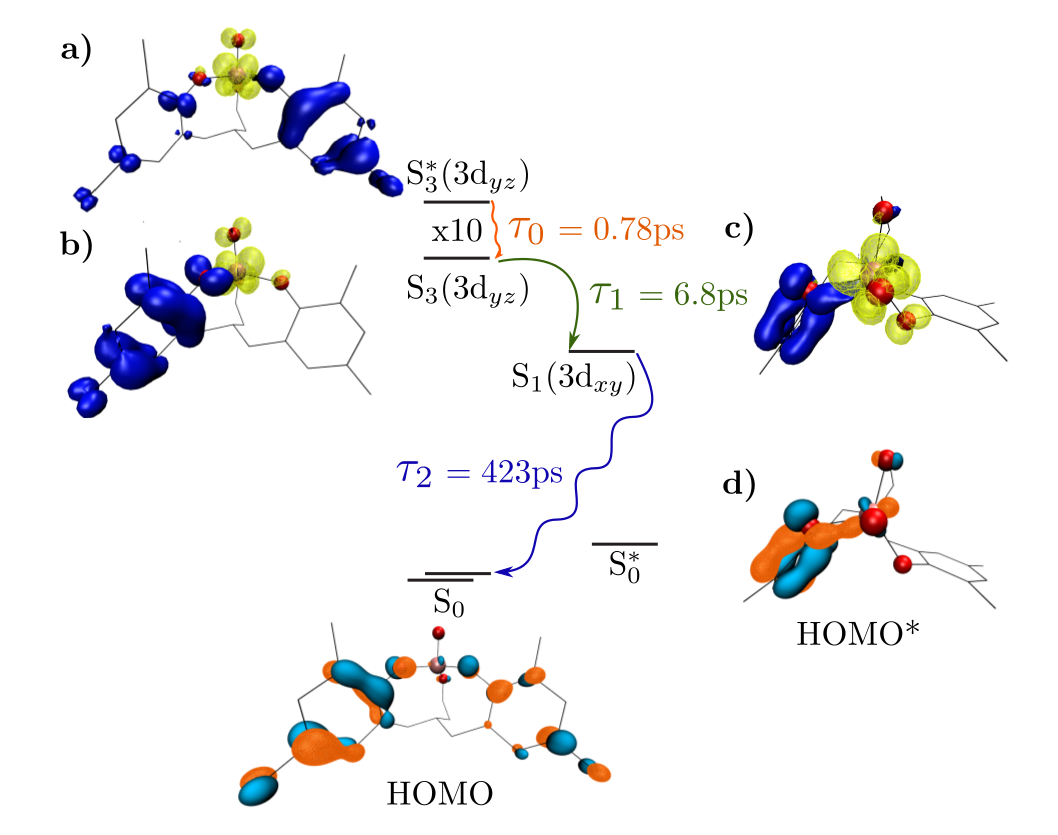
In the search for renewable energy, inorganic nanoscale devices are a promising avenue. Using 3d transition metals is particularly desirable since they are earth abundant and scalable. Shining light (such as sunlight) on these devices causes them to go into an excited state which can be used drive chemical reactions such as water splitting. For these devices to be efficient they should absorb light in the same range of wavelengths output by the sun. Additionally, they should have long excited state lifetimes since they can drive more chemical reactions per photon. Our goal is to determine an appropriate level of theory to predict these properties accurately. This is done by comparing calculated values to experimental data. Once an appropriate level theory is determined, the search for materials can be done computationally which will guide future experiments. In this study, we considered the experimentally synthesized, light absorbing molecule VOLF. We calculated the range of wavelengths that this molecule absorbs light in and found that it absorbs in the visible region. To investigate temperature and environmental effects, molecular dynamics calculations were done in different solvents. Comparing the resulting absorption spectrum calculations to experimental data, we find they agree well. When this molecule absorbs light, there is charge transfer from the outside ligands to the metal center. The absorption of light causes the molecule to go from the ground state to an excited state. The image above shows the excitation and relaxation pathway where the yellow surfaces show where the charge sits after excitation, and the dark blue surfaces indicate where the charge came from. As the molecule relaxes in the excited state, it converts internally to a new excited state. This new excited state does not couple well to the ground state, and the molecule becomes trapped. This trapping leads to a extended excited state lifetime of 423 ps. We have developed a workflow which can be used to predict properties of other light harvesting molecules. In the case of VOLF, our calculations matched experimental data fairly well. This level of theory must be tested against other molecules, however results so far look promising. |