|
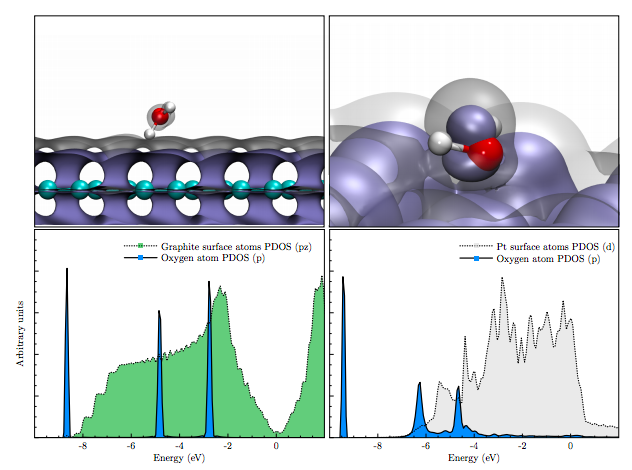
The electrolysis of water is a simple reaction, where hydrogen and oxygen gas is produced from liquid water. This process occurs naturally in photosynthesis, is well studied in the lab, and has been proposed as a means to store energy. In order for this reaction to occur, energy must be input into the system. In the case of photosynthesis, the energy that drives the reaction is from light, but in the lab, one usually immerses two electrodes and applies an electric current through the water. Common electrodes used are Pt, and Graphite. Pt is used because it offers the lowest overpotential, meaning that less energy is needed to drive the reaction. Graphite is less efficient, but it is used because it is cheap, and can be synthetically produced. Since Pt is rare, an active area of research is finding a catalyst that rivals the efficiency, but not the cost. In our study, we try to obtain a nanoscale understanding of electrode-water interfaces by analyzing the differences between liquid water next to Pt, graphite, and graphene slabs. To do so, we calculated Born-Oppenheimer molecular dynamics using state-of-the-art first principles methods. Namely, we used density functional theory with van der Waals interactions, giving the most accurate, large scale description of these interfaces to date. We found that in the case of liquid water next to Pt, an adsorption peak can be seen in the molecular density profile. This peak has not been seen in any other theoretical/experimental study, and is absent in the graphite/graphene interfaces. This adsorption peak is due to water molecules forming covalent bonds with the surface Pt atoms. To further investigate, we then calculated projected density of states for water monomers on the surface as well as the surface atoms, and found that the orbitals sitting on the water molecule broaden in energy, indicating a coupling with the d-band of Pt. This broadening is absent with the water monomer next to the carbon surfaces, which agrees with the molecular density profiles. This chemisorption layer may be a key indicator of an efficient catalyst, and other cheap electrodes with this layer may be promising alternatives to superior rare metals. Future work includes investigating other materials, and further understanding the chemistry of the water-Pt interface using molecular orbital theory. |